By Utah Mining Association
In 2020, the U.S. learned to value many things no one ever expected to lose: full shelves in the grocery store, going to sporting and cultural events, travel and social events. Zoom became a necessity for work, worship and catching up with friends. In short, the high-tech products that made social distancing bearable and kept the restaurant industry afloat through online orders were something we took for granted in the same way we used to not-think about toilet paper. You know you don’t want to live without it, but you don’t think you’ll ever have to.
High-tech products use rare earth elements. The most important application is probably their use in magnets for the coming onslaught of hybrid and electric vehicles. The magnets in these vehicles use neodymium (Nd), praseodymium (Pr), dysprosium (Dy) and terbium (Tb). Other applications include products as diverse as the phone in your pocket, the hard drive in your computer or laptop, medical products, military defense systems and air pollution control. They are even used to make steel alloys. Rare earth metals are needed to make more than 200 products work, even if they only use small quantities. A battery may not look very impressive, but take it out, and how useful is the thing it is supposed to power?
Certain rare earth alloys are extremely hard. They are essential for armored vehicles and projectiles that have been designed to shatter when they hit something.
Imagine navigating the pandemic without rare earth materials. If the pandemic was a nightmare, how much worse would life have been without all the miracle products most people use every day but could not make on their own? You might be able to use innovative methods to grow food, and you could probably figure out how to sew or knit something to wear, especially if you have a stash of yarn. But what if you had to walk instead of drive and could not access Netflix or any other internet staple?
What if you had no national or international news, no way to contact people except face-to-face, and the only way to mow your lawn was with a push mower? Even glass depends on rare earth minerals for polishing, color and optical properties; digital camera lenses in cellphone cameras, for example, may consist of 50% lanthanum. No lanthanum might eventually mean no more selfies — which would be hard on many people who love taking pictures of themselves to share with friends and family.
Rare earth elements are essential in our high-tech society, whether we think about them or not. What exactly are they?
- Rare earth elements are metals.
- Their colors range from silver to gray.
- These elements are soft, ductile, malleable and usually reactive. “Malleable” means you can press or hammer them, and they won’t break; “ductile” means you can make metal wire or thread from them.
- They can become more reactive under some circumstances, such as when you heat them or divide them finely.
A Swedish army lieutenant, Carl Axel Arrhenius, is credited with discovering the first rare earth mineral in 1787. It was black, and he found it in a small-town quarry near Stockholm. (The town was named Yttrby, which is how yttrium got its name in the periodic table.) Isolating the first rare earth element, however, didn’t happen until 1803.
Initially, experts thought rare earths were scarce, which is why they have the name they have. However, that turned out to be a mistake. In general, they are relatively abundant. Cerium is the 25th most common element; thulium and lutetium are the least common.
There are 17 of them: scandium, yttrium and 15 lanthanides. In atomic order, the rare earth elements are listed below:
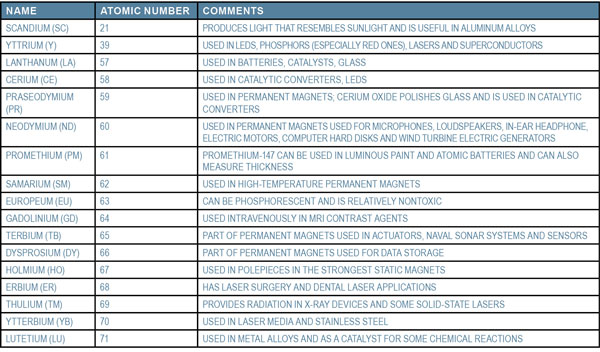
Scandium, yttrium, and lanthanide are in Group IIIB on the periodic table. What that means is they have three electrons in their outer shell that form +3 ions in solution. The lanthanide series, listed below the rest of the periodic table in two rows, form the same ions and can be chemically separated in similar ways. The elements in the first row of the lanthanide series are the ones that are considered to be rare earth elements.
The term “rare earths” refers to oxides that have rare earth elements in them. (An oxide is a binary chemical compound that occurs when oxygen atoms combine with other elements. For example, water and carbon dioxide are both oxides.) Oxides can be acidic, basic, amphoteric (that is, it has both acid and base reactions), and neutral (not acidic or basic).
Lanthanides are interesting because they have photophysical properties such as long-lived luminescence. You can make highly luminescent complexes from them.
Separating rare earth elements from other elements is tricky. Ordinary chemical methods don’t work because their chemical properties are too similar. Between 1787 and 1947, some scientists spent their entire career trying to get a 99% pure rare earth; the most common method was through fractional crystallization that focused on differences in solubility. In 1947, two different scientists and their colleagues came up with successful new methods that depend on ion exchanges: Gerald Boyd at Oak Ridge National Laboratory and Frank Harold Spedding at the Ames Laboratory in Iowa.
The USGS Mineral Commodity Summary in 2017 listed the following usage categories for rare earth elements:
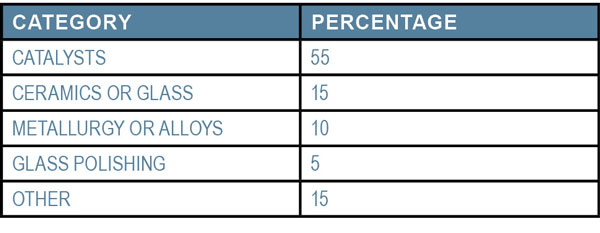
The need for rare earths first surfaced in the 1960s when people began buying color televisions. However, demand for rare earth elements has increased dramatically since 2000 because of their applications in cellphones and computers. Rechargeable batteries often use them, too. Electric and hybrid vehicles use substantially more rare earth elements than cellphones and computers. Even though the market is fairly saturated with cellphones and computers, the shift from gas-powered engines to electric and hybrid ones will unleash an even greater demand in the future, especially for neodymium and praseodymium.
Which countries produce rare earths? China is the current leader. That is because China created a monopoly. In the late 1980s and early 1990s, China first got into the market and sold rare earths at such low prices that U.S. mines couldn’t compete and had to close. It controlled 95% of production in 2010. China then cut back its exports and raised its rare earth prices — more than 500% in some cases. Also, China often threatens to cut off rare-earth exports to the U.S. and U.S. allies. The U.S., Australia, Russia, Thailand, Malaysia and other places sensibly decided to get back into mining rare earths when faced with these new circumstances.
Diversification is as important in mining as it is in the stock market, PPE and grocery essentials. If one source goes away, you can still rely on others to meet your needs.
Domestic production of rare earths matters because (as the U.S. has learned during the last year) long supply chains are also brittle supply chains. There’s just no substitute for being able to produce goods within national borders.
Mining rare earths responsibly within the U.S. matters. You may not have realized how much of a role it plays in maintaining your day-to-day life, but without it, 2020 would have been exponentially worse.